Stracyfic
Patient Stratification by Standardization of the Image-based Sweat Test for Cystic Fibrosis for use in Clinical Routine
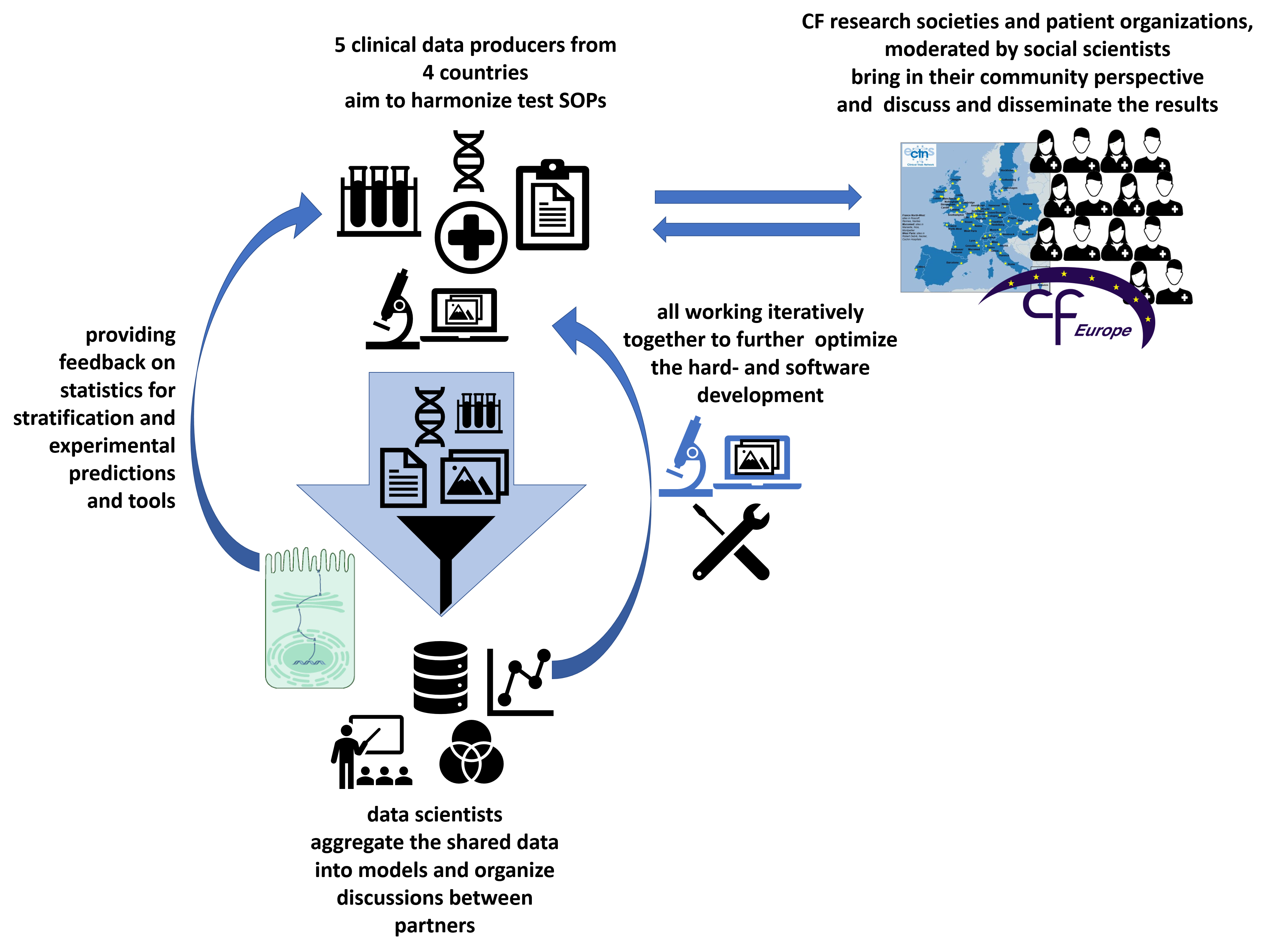
Aims of this collaborative project
The demonstration of a salty sweat has long been used to diagnose cystic fibrosis (CF), a rare disorder affecting 1 in 2,500 live births and associated with high morbidity and mortality. CF represents the most frequent recessive genetic disorder. CF is caused by CFTR mutations resulting in loss-of-function of the CFTR protein that mainly acts as a chloride channel. It leads to dramatic transepithelial ion and water transport abnormalities and produces a thick mucus obstructing airways and duct lumens of exocrine glands. Beyond complex treatments, mainly symptomatic, CFTR modulators have recently been developed to mitigate the mutation effects; there is however still no cure for CF. Moreover, there is an unmet need of validated biomarkers of CFTR function to quantify the remaining CFTR activity, e.g. to classify the level of the base defect and later assess the efficacy of target therapies. We aim at developing a usable common standard for the required experiments, the automated analysis via software and providing the experimental hardware setups for an easy dissemination of the technique to other sites. Networking transnational dedicated computer scientists, biologists, care givers and social scientists, the Stracyfic project addresses the aims of the ERAPerMed call by bringing a novel biomarker to a translational use level. Thus, further improving and standardizing the test setup and analysis across different sites and countries. Starting with partners from Belgium, France, Germany and Italy, this includes the generation and distribution of adequate training/teaching material. Social and ethical and also patient organizations are included to assess the impact of the transformation and guide the process. Stracyfic will offer a novel strategy to better classify and monitor patients by their individual level of the disease-causing effect. Enabling better detection as well as better management of the disease in the long run.
Required steps for the measurements
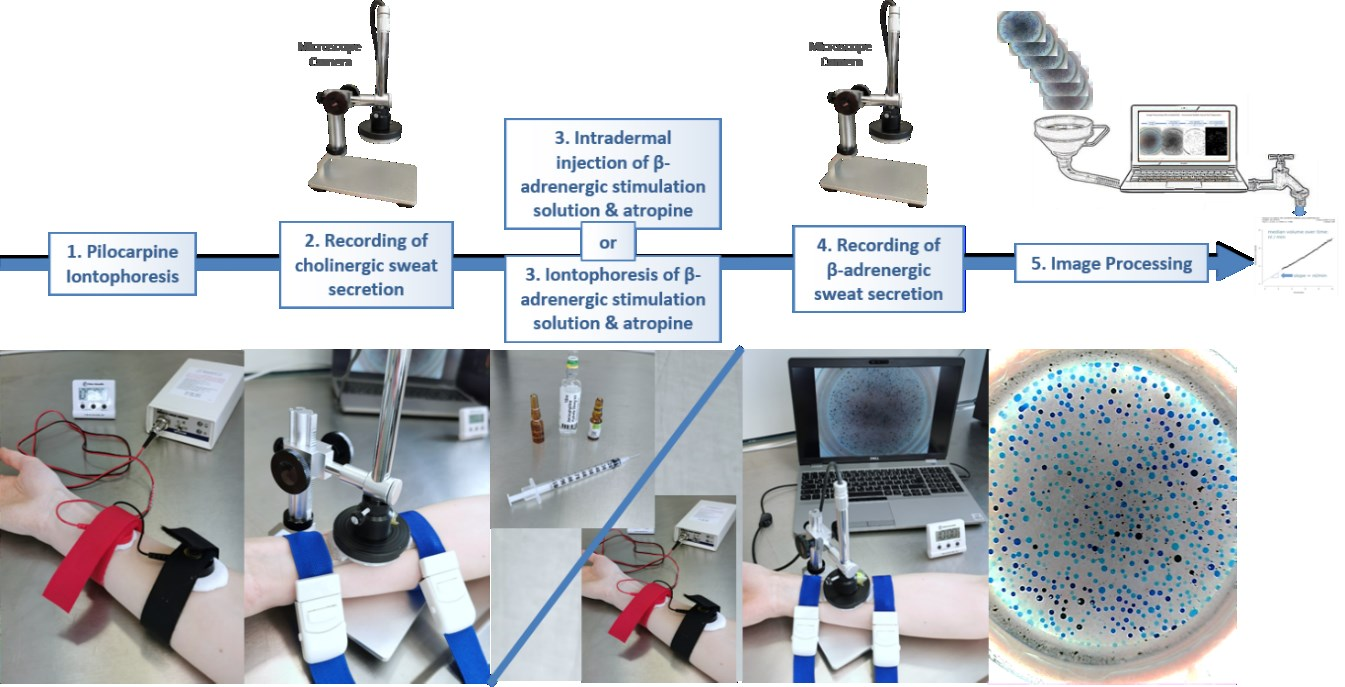
Hardware setup for capturing the images

Project partners
The hard- and software is being developed in Göttingen and tested transnationally by our project partners with cohorts from Brussels, Hannover, Milan and Paris. The project aims also to define a common standard operating procedure for the stimulation of the required sweat response, which is developed in the team with our clinical partners. Social scientist and patient organisations will add their viewpoints to the teams perspective.
Coordinator - Germany, Göttingen
Department of Medical Bioinformatics (UMG) - Group of Chemoinformatics and Imaging
Dr. Manuel Nietert
Associated Site:
Hannover Medical School (MHH) - Dr. med. Sophia Pallenberg & PD. Dr. Anna Maria Dittrich -
Clinic for Pediatric Pneumology, Allergology and Neonatology
Clinical Partner - Belgium, Brussels
Prof. Dr. Sophie Gohy - Cliniques universitaires Saint-Luc, Université catholique de Louvain
Clinical Partner - France, Paris
Prof. Dr. Isabelle Gaudelus-Sermet - Institut national de la santé et de la recherche médicale (INSERM)
Clinical Partner - Italy, Milan
Prof. Dr. med. Andrea Gramegna - IRCCS Ca’ Granda Ospedale Maggiore Policlinico
Social Scientist Partner - Germany, Hamburg
Prof. Dr. Wöhlke Sabine - Hamburg University of Applied Science
Associated Partner Patient Organisation: CF-Europewith the German patient organisation Muko e.V.- contact for patient organisation Dr. Sylvia Hafkemeyer
Funding
The project is funded as part of a joint transnational call initative ERA PerMed in 2022. The project duration is 01/06/2023 to 31/05/2026. Our institution is funded by the German Federal Ministry of Health (Bundesministerium für Gesundheit), while our project partners receive their own funding from their own countries funding agencies.


Press releases
Press release from the Mukoviszidose Institut – gemeinnützige Gesellschaft für Forschung und Therapieentwicklung mbH:
https://idw-online.de/de/news818593
That might also interest you